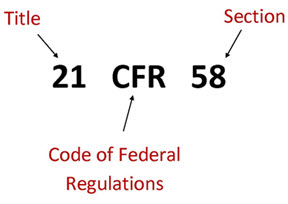
Book M2: 2022 Mini Pocket-Sized (3" x 5") Code of Federal Regulations – Clinical Research Resources, LLC

CODE OF FEDERAL REGULATIONS TITLE 21 Food And Drugs BUDGET EDITION 2018 PARTS 1-99: CFR TITLE 21: FEDERAL REGISTER, OFFICE OF THE: 9781731153425: Amazon.com: Books

GMP Regulation Handbook: General, Finished Pharmaceuticals, 21 CFR Parts 210 & 211 | ISPE | International Society for Pharmaceutical Engineering

CFR 21, Parts 300 to 499, Food and Drugs, April 01, 2017 (Volume 5 of 9): Office of the Federal Register (CFR): 9781298709035: Amazon.com: Books

National Hot Sauce Day and Food Regulations – Pic of the Week | In Custodia Legis: Law Librarians of Congress
.jpg)